- Teacher: naomy chepkoech
naomychepkoech.gnomio.com
-
naomy
Welcome to your new Gnomio site
Now, you are in control!
Moodle is an open-source Learning Management System (LMS) that provides educators with the tools and features to create and manage online courses. It allows educators to organize course materials, create quizzes and assignments, host discussion forums, and track student progress. Moodle is highly flexible and can be customized to meet the specific needs of different institutions and learning environments.
Moodle supports both synchronous and asynchronous learning environments, enabling educators to host live webinars, video conferences, and chat sessions, as well as providing a variety of tools that support self-paced learning, including videos, interactive quizzes, and discussion forums. The platform also integrates with other tools and systems, such as Google Apps and plagiarism detection software, to provide a seamless learning experience.
Moodle is widely used in educational institutions, including universities, K-12 schools, and corporate training programs. It is well-suited to online and blended learning environments and distance education programs. Additionally, Moodle's accessibility features make it a popular choice for learners with disabilities, ensuring that courses are inclusive and accessible to all learners.
The Moodle community is an active group of users, developers, and educators who contribute to the platform's development and improvement. The community provides support, resources, and documentation for users, as well as a forum for sharing ideas and best practices. Moodle releases regular updates and improvements, ensuring that the platform remains up-to-date with the latest technologies and best practices.
Links of interest:
(You can edit or remove this text)
Available courses
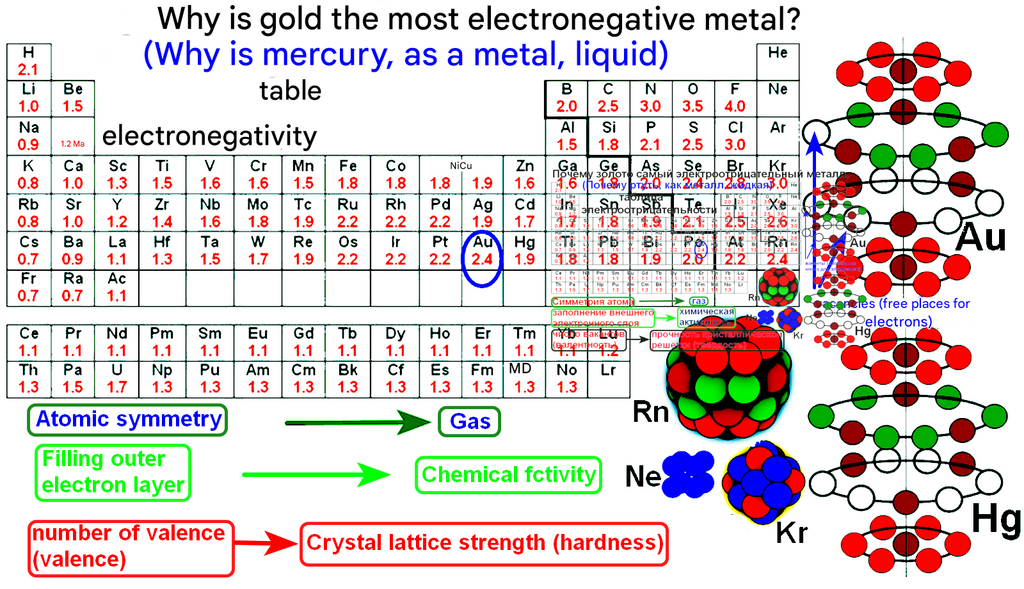
Physical Chemistry is the branch of chemistry that uses the principles and techniques of physics to understand chemical systems. It seeks to answer the fundamental questions:
-
What makes chemical processes happen? (Thermodynamics)
-
How fast do they go? (Kinetics)
-
How is the structure of a molecule connected to its properties and behavior? (Quantum Mechanics & Spectroscopy)
- Teacher: naomy chepkoech

Inorganic Chemistry is a branch of chemistry that deals with the properties and behavior of inorganic compounds. This course provides a comprehensive overview of the fundamental concepts and principles of inorganic chemistry, including:
-
Chemical Bonding: Explore the nature of ionic, covalent, and metallic bonds, and understand molecular geometry and hybridization.
-
Coordination Chemistry: Study coordination compounds, their structures, bonding theories, and the role of ligands.
-
Main Group Elements: Examine the chemistry of the main group elements, including their reactivity, trends in the periodic table, and applications.
-
Transition Metals: Investigate the unique properties of transition metals, including their electronic configurations, oxidation states, and their role in catalysis.
-
Bioinorganic Chemistry: Understand the significance of metals in biological systems, including metalloproteins and metalloenzymes.
-
Solid State Chemistry: Explore the structure, properties, and synthesis of inorganic solids, including crystallography and defects.
Learning Outcomes:
By the end of this course, students will be able to:
- Explain the fundamental principles of inorganic chemistry and apply them to solve problems.
- Analyze the structure and bonding of inorganic compounds using various theories.
- Demonstrate knowledge of the role of inorganic substances in biological and industrial processes.
- Conduct laboratory experiments to synthesize and characterize inorganic compounds.
- Teacher: naomy chepkoech
Here's a summary of the key concepts and topics typically covered in an undergraduate organic chemistry course:
I. Fundamentals of Structure and Bonding:
-
Atomic Structure and Hybridization: Understanding atomic orbitals (s, p), how they combine to form hybrid orbitals (sp, sp$^2$, sp$^3$), and the resulting molecular geometries (linear, trigonal planar, tetrahedral). This is crucial for predicting molecular shape and reactivity.
-
Covalent Bonding: The nature of single, double, and triple bonds (sigma and pi bonds).
-
Lewis Structures and Resonance: Drawing valid Lewis structures and understanding resonance theory to describe electron delocalization and its impact on stability and reactivity.
-
Acids and Bases: Bronsted-Lowry and Lewis acid-base theories, pKa values, and predicting acid-base reaction outcomes.
-
Intermolecular Forces: Understanding how hydrogen bonding, dipole-dipole interactions, and London dispersion forces affect physical properties like boiling point and solubility.
II. Introduction to Organic Compounds and Functional Groups:
-
Hydrocarbons:
-
Alkanes: Nomenclature (IUPAC and common names), conformations (Newman projections, chair conformations of cycloalkanes), and their relatively low reactivity (free radical halogenation).
-
Alkenes: Nomenclature, E/Z isomerism, and characteristic addition reactions (electrophilic addition, hydrogenation, halogenation, hydration, hydrohalogenation, ozonolysis).
-
Alkynes: Nomenclature, and characteristic addition reactions.
-
-
Functional Groups: Recognizing and understanding the properties and reactivity associated with common functional groups, which are specific arrangements of atoms that dictate chemical behavior (e.g., alcohols, ethers, alkyl halides, amines, aldehydes, ketones, carboxylic acids, esters, amides, nitriles, etc.).
-
Isomerism:
-
Constitutional (Structural) Isomers: Compounds with the same molecular formula but different connectivity.
-
Stereoisomers: Compounds with the same connectivity but different spatial arrangements.
-
Chirality and Stereocenters: Identifying chiral molecules and stereocenters.
-
Enantiomers and Diastereomers: Distinguishing between different types of stereoisomers.
-
R/S Configuration: Assigning absolute configuration to stereocenters.
-
-
III. Organic Reactions and Mechanisms:
A major focus of organic chemistry is understanding reaction mechanisms, which are the step-by-step pathways of electron movement. Key reaction types include:
-
Substitution Reactions:
-
Nucleophilic Substitution (SN1, SN2): Understanding the factors influencing these reactions (steric hindrance, solvent, leaving group, nucleophile strength).
-
Electrophilic Aromatic Substitution (EAS): Reactions of benzene and other aromatic compounds.
-
-
Elimination Reactions (E1, E2): Understanding the factors influencing these reactions and their competition with substitution.
-
Addition Reactions: Primarily seen with alkenes, alkynes, and carbonyl compounds.
-
Oxidation and Reduction Reactions: Recognizing common oxidizing and reducing agents and their application in organic synthesis.
-
Rearrangement Reactions: Reactions where atoms or groups migrate within a molecule.
-
Pericyclic Reactions: Concerted reactions involving cyclic transition states (e.g., Diels-Alder reaction).
-
Radical Reactions: Reactions involving intermediates with unpaired electrons.
IV. Spectroscopy and Structure Determination:
-
Infrared (IR) Spectroscopy: Identifying functional groups based on characteristic vibrational frequencies.
-
Nuclear Magnetic Resonance (NMR) Spectroscopy (Proton and Carbon-13): Determining the carbon-hydrogen framework of a molecule and the electronic environment of atoms.
-
Mass Spectrometry (MS): Determining molecular weight and fragmentation patterns to deduce structural information.
-
UV-Vis Spectroscopy: Used for conjugated systems.
V. Synthesis:
-
Retrosynthesis: Working backward from a target molecule to design a synthetic pathway.
-
Multistep Synthesis: Combining various reactions to synthesize complex organic molecules.
VI. Advanced Topics (often in a second semester):
-
Chemistry of Carbonyl Compounds:
-
Aldehydes and Ketones: Nucleophilic addition reactions.
-
Carboxylic Acids and Derivatives (Esters, Amides, Acid Chlorides, Anhydrides): Nucleophilic acyl substitution reactions.
-
Alpha-Carbon Chemistry: Enols, enolates, aldol condensation, Claisen condensation.
-
-
Amines: Basicity, synthesis, and reactions.
-
Conjugated Systems: Dienes, resonance, and pericyclic reactions (e.g., Diels-Alder).
-
Aromatic Compounds: Aromaticity, electrophilic aromatic substitution (directing effects, activating/deactivating groups).
-
Special Topics: Depending on the course, this might include carbohydrates, amino acids, proteins, lipids, polymers, or organometallic chemistry.
Learning Outcomes:
Upon successful completion of an organic chemistry course, students are typically expected to be able to:
-
Draw and interpret various types of organic structures (Lewis, condensed, bond-line).
-
Name organic compounds using IUPAC nomenclature.
-
Understand and apply principles of structure, bonding, and molecular forces to predict physical and chemical properties.
-
Predict and explain reaction products, including stereochemistry.
-
Propose logical, step-by-step reaction mechanisms using arrow-pushing notation.
-
Design multi-step synthetic routes for target molecules.
-
Interpret spectroscopic data (IR, NMR, MS) to determine unknown structures.
-
Apply concepts of stability, acidity, and basicity to explain reactivity.
Organic chemistry is often challenging due to the sheer volume of reactions and mechanisms. However, it's also highly logical, emphasizing pattern recognition and mechanistic reasoning rather than rote memorization. Mastering the fundamental concepts and practicing problem-solving are key to success.
- Teacher: naomy chepkoech
